
Synaptic neurochemistry laboratory
Group home page at UiO
Group home page (folk.uio.no)
Brain and muscle energy: Linda H. Bergersen group
Pages at folk.uio.no
Neuro- and gliotransmitters: Vidar Gundersen group
Pages at folk.uio.no
Associated biotek group: Farrukh A. Chaudhry
The group’s main interests are the mechanisms underlying synaptic transmission and gliotransmission, and the role of metabolism and energy supply for the function of gray and white matter. These mechanisms are studied in normal and pathological conditions, and during ontogenetic development and ageing.
Challenges
Recent research by our group (see Achievements) has opened possibilities for studying in depth aspects of nervous system functions in health and disease. Important aspects are how nerve endings provide glutamate for synaptic release and how they recover released glutamate for reuse, as well as how synapses provide energy for synaptic transmission and how astrocytes can modulate neuronal function. Our main aim is to study synaptic function under physiological conditions and to investigate how the factors contributing to normal signalling are altered in disease, identifying new therapeutic strategies.
Projects
- Identification of gliotransmitters and their roles in neuron-glia communication.
- Role of metabolic precursors of glutamate, including glutamine, for keeping up synaptic release.
- Interplay of glutamate with other neurotransmitters (e.g. aspartate, GABA, dopamine), including experimental models of neurological disease (e.g. Parkinson's disease, epilepsy, ADHD).
- Roles of lactate in synaptic transmission and myelinisation studied in monocarboxylate transporter knock-out mice, as well as in experimental models of heart failures and epilepsy.
- Synaptic changes during ontogenetic development and in animals with deficient DNA repair.
Achievements
Glutamamine transporters, SN (Cell 1999, EMBO J 2001, Eur J Neurosci 2002, Glia 2003, J Am Soc Nephrol 2005) and SA/SAT (PNAS 2000, J Neurosci 2002, J Cell Biol 2002) were molecularly identified and characterized. A role of glutamine has been defined for normal synaptic function (J Neurochem 2008) as well as dendritic retrograde signaling (Cereb Cortex 2009c), and a potential target uncovered in Alzheimer’s disease (Neurochem Res 2008). The ultrastructural localization of monocarboxyltate transporters (Cereb Cortex 2005, Neuroscience 2007a) as well as identification of glutamate transporters in glia (Glia 2008) and in nerve endings (Neuroscience 2008) provides new approaches to understanding brain function. The identification of proteins, VGLUT1-3 (Neuron 2001, PNAS 2002), that pump glutamate into synaptic vesicles allows the packaging of the transmitter to be characterised in health and disease (J Comp Neurol 2004, 2006, 2007) and modified by gene knock-out (Science 2004). Astrocytes, triggered by e.g. purinergic receptors (Eur J Neurosci 2007), release glutamate from VGLUT containing vesicles to enhance synaptic efficacy (Nature Neurosci 2004, 2007, Neuroscience 2009a). The observations that astrocytes and even non-neural cells (J Cell Sci 2004, J Lipid Res 2007) store and can release neurotransmitter amino acids in a way resembling synaptic release, and that oligodendrocytes have NMDA type glutamate receptors (Nature 2005), together with findings that glutamate and other neuroactive substances can be co-released from nerve endings (Eur J Neurosci 2003, Molec Neurosci 2004, Cereb Cortex 2009a), including at the neuromuscular junction (Neuroscience 2007b), suggest novel ways of intercellular communication and potential drug targets. Observations in synapsin knock-out mice that develop epilepsy (Neuroscience 2005, Cereb Cortex 2009b) and in a rat model of ADHD (Neuroscience 2009b) implicate anomalous glutamate signalling in these diseases. Ionotrophic glutamate receptors are implicated in nociception (Mol Neurobiol 2009), and mediate signals that position mitochondria where they are most needed, i.e. at the postsynaptic site of active synapses (Neuron 2009). Inducible expression of a mutated mitochondrial UNG1 DNA repair enzyme in forebrain neurons caused generation of apyrimidinic mDNA and neuronal impairment including reduced size of synaptic contacts (Mol Cell Mol 2010). Eccentric muscle actions cause ultrastructural changes in human subjects (J Appl Physiol 2009 a, b). Increased MCT1 expression after ischemia and reperfusion restore cardiac pH through lactate export (Life Sci 2009). Review of the literature underlines a role for vesicular release of glutamate from astrocytes in synaptic transmission (Neuroscience 2009a). Symposia were published, on the glutamate synapse (Neuroscience 2009c), and on synaptic plasticity and memory (Neuroscience 2009d).
Publications
Acting group leader
 Associate Professor Linda Hildegard Bergersen
Associate Professor Linda Hildegard Bergersen
Department of Anatomy & CMBN
University of Oslo
PO Box 1105 Blindern
NO-0317 Oslo
Norway
Tel: +47 22851496 or +47 97032049
Fax: +47 22851278
E-mail: l.h.bergersen@medisin.uio.no
Founding group leader
 Professor
Jon Storm-Mathisen
Professor
Jon Storm-Mathisen
Department of Anatomy & CMBN
University of Oslo
PO Box 1105 Blindern
NO-0317 Oslo
Norway
Tel: +47 22851258 or +47 97193044
Fax: +47 22851278
E-mail: jon.storm-mathisen@medisin.uio.no
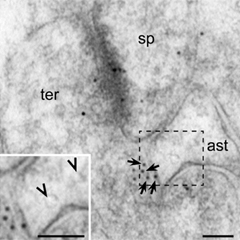
Electron micrograph showing NMDA receptor subunits NR2B (immunogold particles) at the synapse as well as in extrasynaptic membranes (arrows) of nerve terminals (ter) making asymmetric synapses with dendritic spines (sp) in the dentate molecular layer. NR2B particles face astrocytic processes (ast) that contain synaptic-like microvesicles (SLMVs, arrowheads in inset) in close proximity. Scale bars, 100 nm.
Nature Neuroscience
Latest publications
Gene expression of glutamate metabolizing enzymes in the hippocampal formation in human temporal lobe epilepsy
Epilepsia, 54 (2), 228-38
PubMed 23384343
Inflammatory markers CD11b, CD16, CD66b, CD68, myeloperoxidase and neutrophil elastase in eccentric exercised human skeletal muscles
Histochem Cell Biol (in press)
PubMed 23224298
Vesicular uptake and exocytosis of L-aspartate is independent of sialin
FASEB J, 27 (3), 1264-74
PubMed 23221336
Pre- and postsynaptic localization of NMDA receptor subunits at hippocampal mossy fibre synapses
Neuroscience, 230, 139-50
PubMed 23159309
Stratigraphic context and paleoenvironmental significance of minor taxa (Pisces, Reptilia, Aves, Rodentia) from the late Early Pleistocene paleoanthropological site of Buia (Eritrea)
J Hum Evol, 64 (1), 83-92
PubMed 23159190
Valproate causes reduction of the excitatory amino acid aspartate in nerve terminals
Neurosci Lett, 527 (2), 100-4
PubMed 22963924
Hippocampal adult neurogenesis is maintained by Neil3-dependent repair of oxidative DNA lesions in neural progenitor cells
Cell Rep, 2 (3), 503-10
PubMed 22959434
Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy
J Neuropathol Exp Neurol, 71 (9), 814-25
PubMed 22878665
Neuroscience: The wrap that feeds neurons
Nature, 487 (7408), 435-6
PubMed 22836992
Oxygen consumption and blood flow coupling in human motor cortex during intense finger tapping: implication for a role of lactate
J Cereb Blood Flow Metab, 32 (10), 1859-68
PubMed 22781333
PO Box 1105 Blindern, NO-0317 Oslo, Norway. Tel: +47 22851528. Fax: +47 22851488